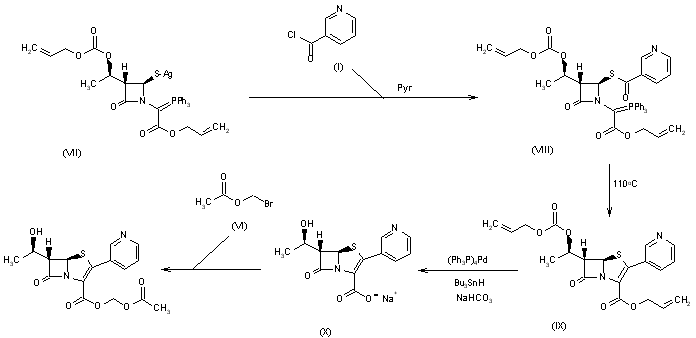
Figure 1 from Palladium-catalyzed chemoselective allylic substitution, Suzuki-Miyaura cross-coupling, and allene formation of bifunctional 2-B(pin)-substituted allylic acetate derivatives. | Semantic Scholar
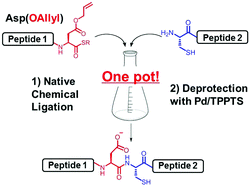
Efficient peptide ligation between allyl-protected Asp and Cys followed by palladium-mediated deprotection - Chemical Communications (RSC Publishing)

Facile and selective cleavage of allyl ethers, amines and esters using polymethylhydrosiloxane-ZnCl2/Pd(PPh3)4
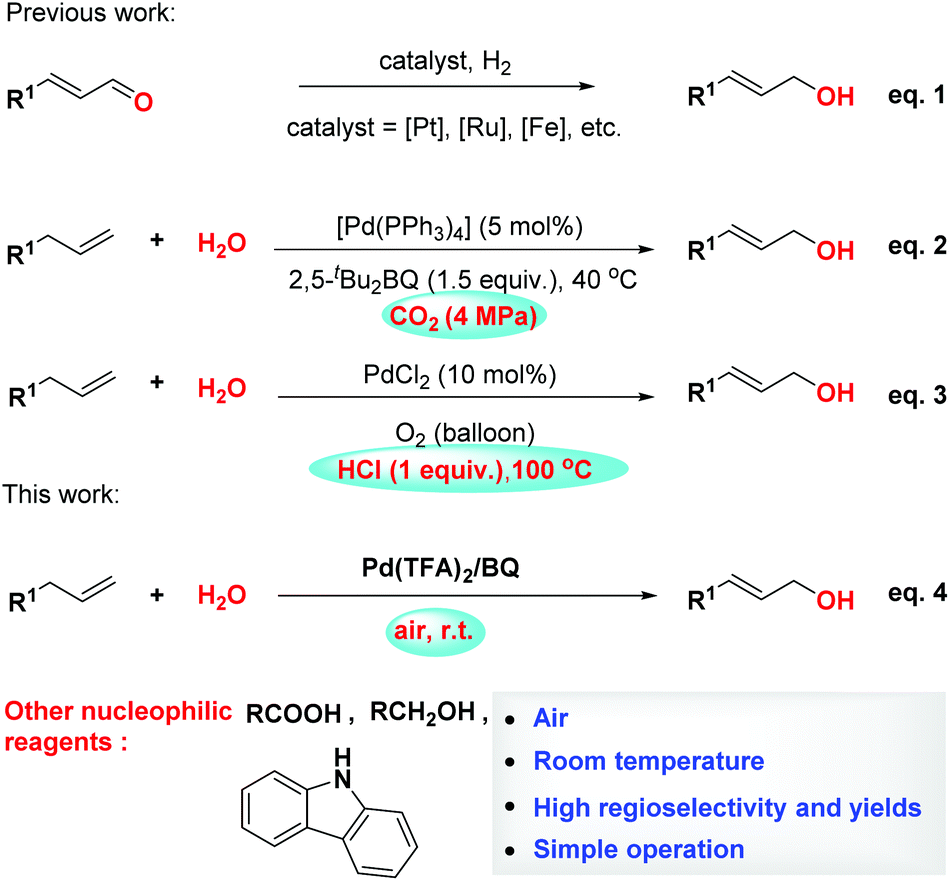
Palladium-catalyzed allylic C–H oxidation under simple operation and mild conditions - Organic & Biomolecular Chemistry (RSC Publishing)
A Mechanistic Study of Direct Activation of Allylic Alcohols in Palladium Catalyzed Amination Reactions
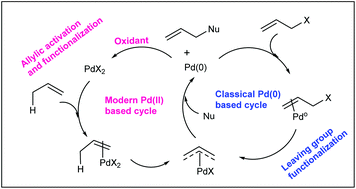
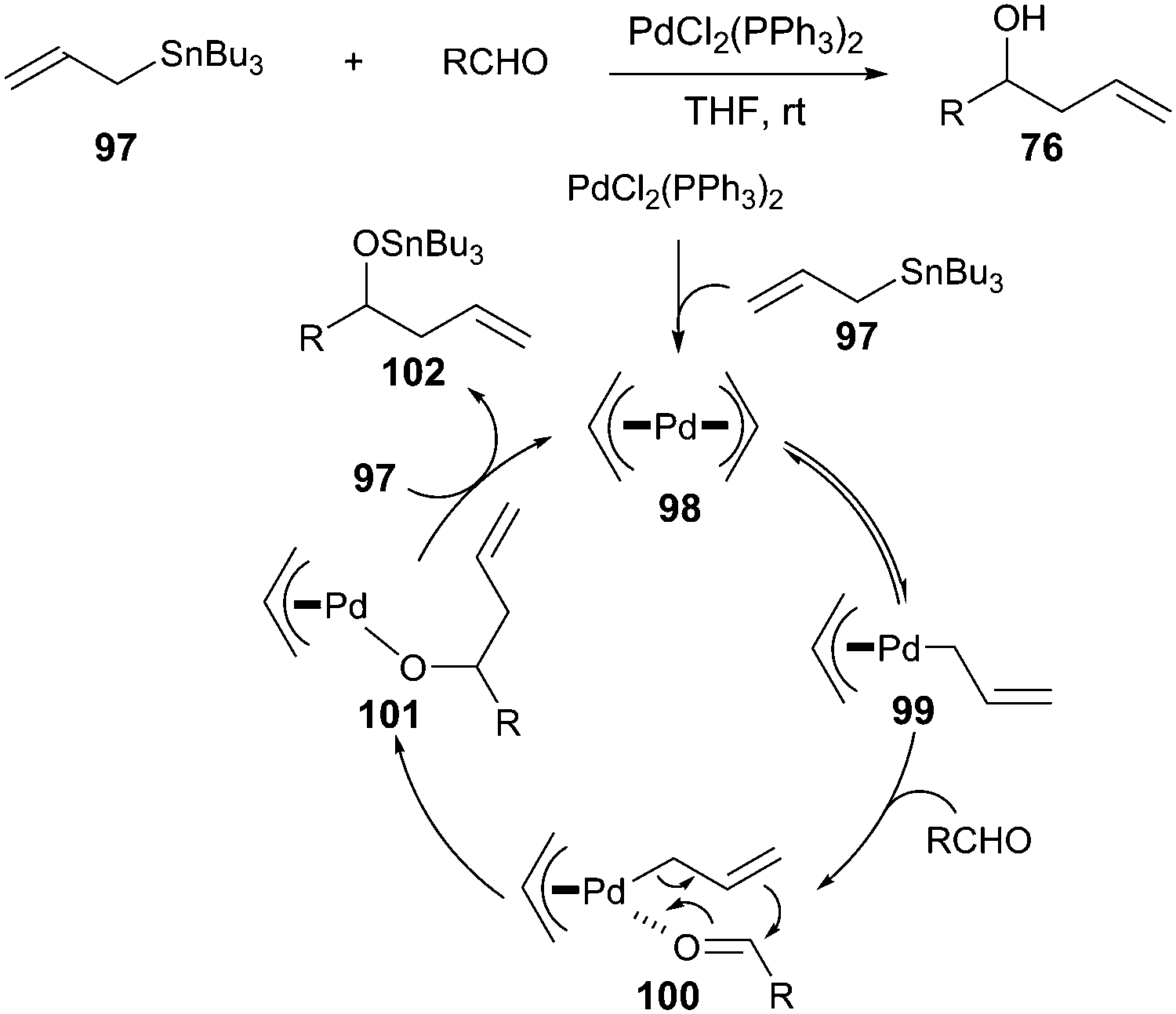
Catalytic allylic functionalization via π-allyl palladium chemistry - Organic & Biomolecular Chemistry (RSC Publishing)

Microwave‐assisted cleavage of Alloc and Allyl Ester protecting groups in solid phase peptide synthesis - Wilson - 2016 - Journal of Peptide Science - Wiley Online Library
Research Letter A Novel, One-Step Palladium and Phenylsilane Activated Amidation from Allyl Ester on Solid Support
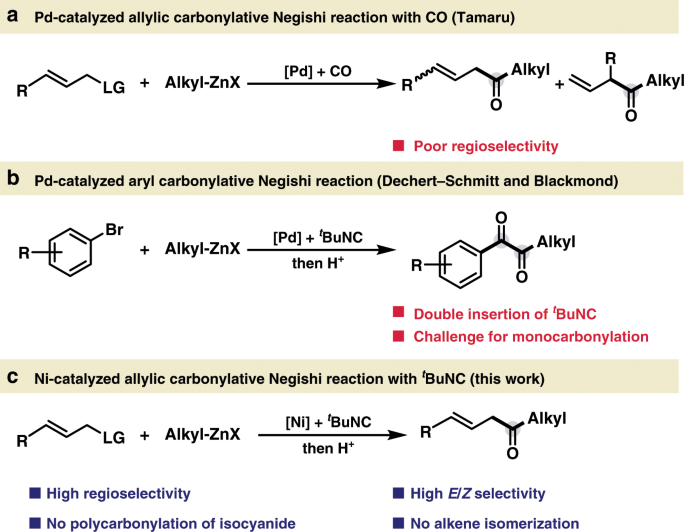
Nickel-catalyzed allylic carbonylative coupling of alkyl zinc reagents with tert -butyl isocyanide | Nature Communications
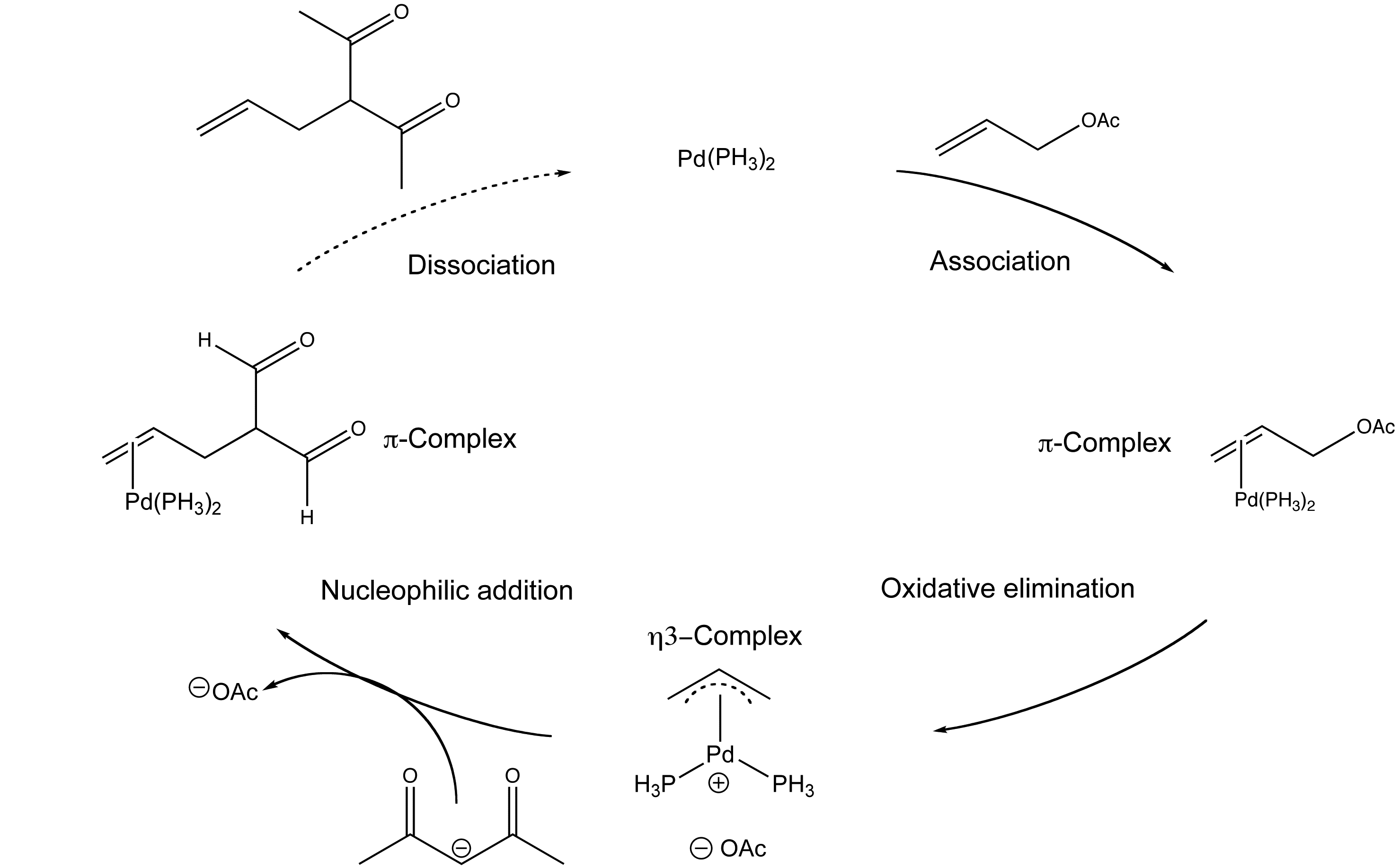
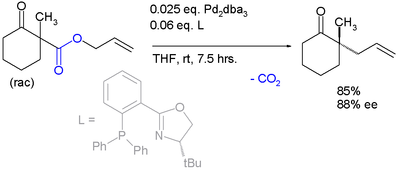
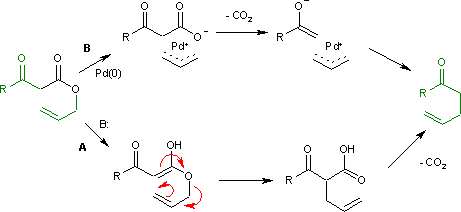
Palladium‐Catalyzed Decarboxylative Asymmetric Allylic Alkylation: Development, Mechanistic Understanding and Recent Advances,Advanced Synthesis & Catalysis - X-MOL
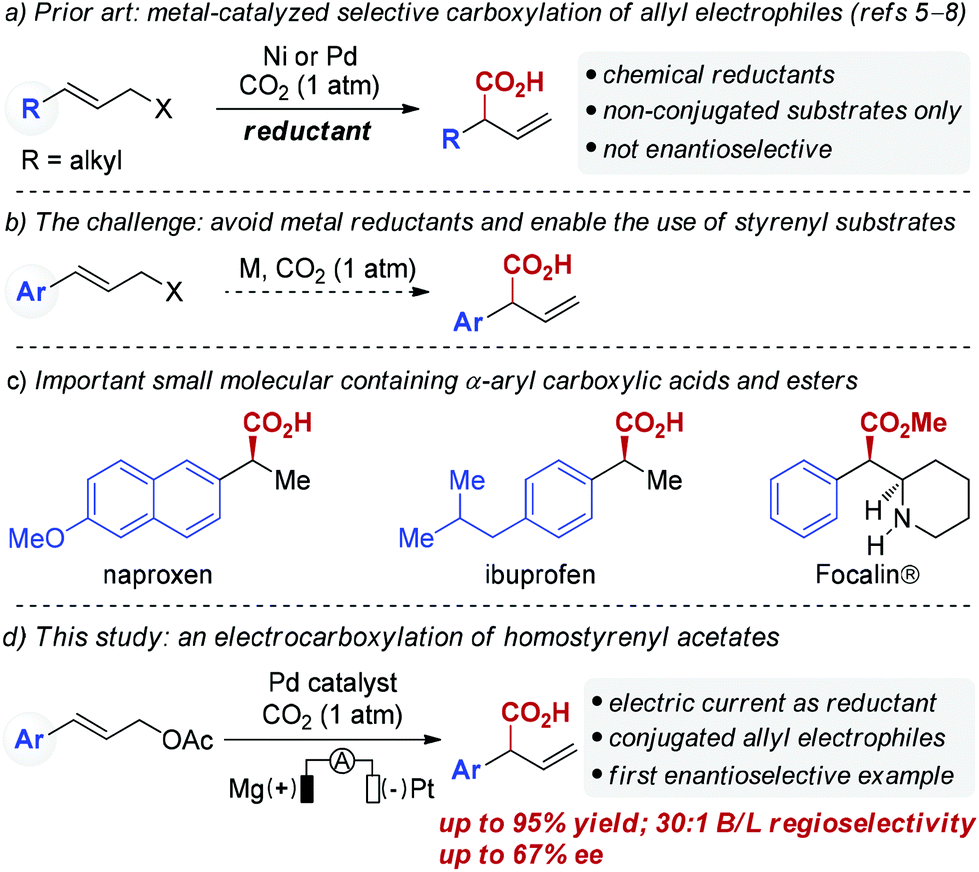
Palladium-catalyzed reductive electrocarboxylation of allyl esters with carbon dioxide - Organic Chemistry Frontiers (RSC Publishing)

Safe Removal of the Allyl Protecting Groups of Allyl Esters using a Recyclable, Low‐Leaching and Ligand‐Free Palladium Nanoparticle Catalyst - Takagi - 2015 - Advanced Synthesis & Catalysis - Wiley Online Library

Microwave‐assisted cleavage of Alloc and Allyl Ester protecting groups in solid phase peptide synthesis - Wilson - 2016 - Journal of Peptide Science - Wiley Online Library

Figure 1 from Amide α,β-Dehydrogenation Using Allyl-Palladium Catalysis and a Hindered Monodentate Anilide. | Semantic Scholar
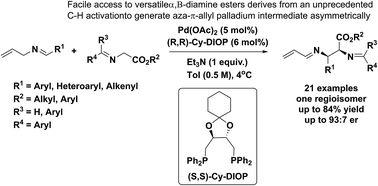
Pd-catalyzed asymmetric allylic alkylations via C–H activation of N-allyl imines with glycinates - Chemical Science (RSC Publishing)