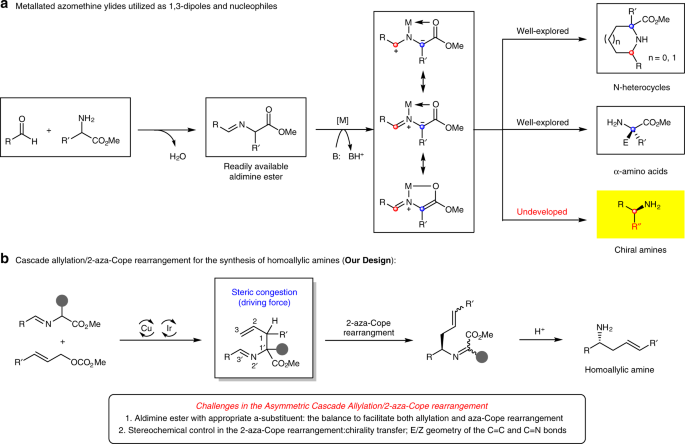
Synergistic catalysis for cascade allylation and 2-aza-cope rearrangement of azomethine ylides | Nature Communications

Table 2.5 from Part A: Palladium(II)-Catalyzed Enantioselective Saucy-Marbet Claisen Rearrangement of Propargyloxy indoles to Quaternary Oxindoles and Spirocyclic Lactones. Part B: The Regioselective Oxidative Coupling of Phenols . | Semantic Scholar

Copper(II)- and Palladium(II)-Catalyzed Enantioselective Claisen Rearrangement of Allyloxy- and Propargyloxy-Indoles

Catalysis of the Claisen Rearrangement of Aliphatic Allyl Vinyl Ethers - Hiersemann - 2002 - European Journal of Organic Chemistry - Wiley Online Library
A continuous-flow resonator-type microwave reactor for high-efficiency organic synthesis and Claisen rearrangement as a model re

Table 2.2 from Part A: Palladium(II)-Catalyzed Enantioselective Saucy-Marbet Claisen Rearrangement of Propargyloxy indoles to Quaternary Oxindoles and Spirocyclic Lactones. Part B: The Regioselective Oxidative Coupling of Phenols . | Semantic Scholar

Table 2.2 from Part A: Palladium(II)-Catalyzed Enantioselective Saucy-Marbet Claisen Rearrangement of Propargyloxy indoles to Quaternary Oxindoles and Spirocyclic Lactones. Part B: The Regioselective Oxidative Coupling of Phenols . | Semantic Scholar
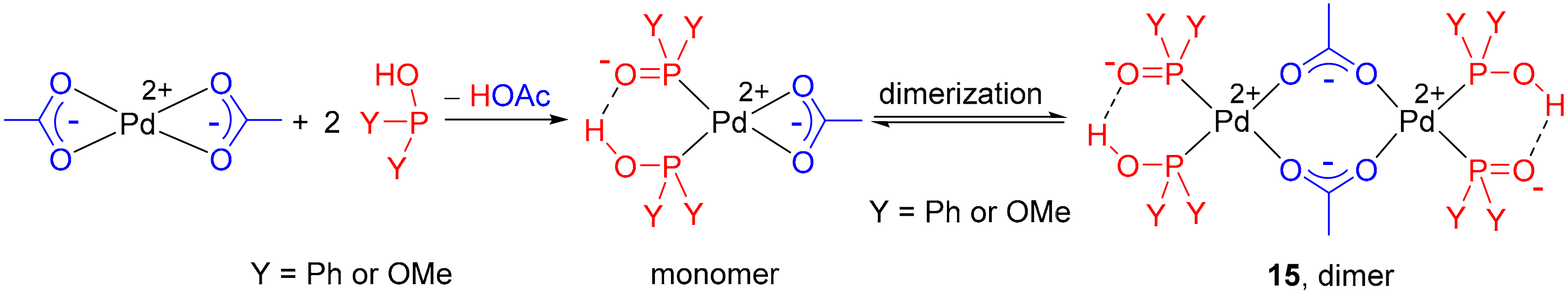
Molecules | Free Full-Text | Focusing on the Catalysts of the Pd- and Ni- Catalyzed Hirao Reactions | HTML

Regiochemical control in the Pd(II)-catalyzed claisen rearrangement via in situ enol ether exchange - ScienceDirect

Figure 1.2 from Part A: Palladium(II)-catalyzed enantioselective Saucy-Marbet Claisen rearrangement of propargyloxy indoles to quaternary oxindoles and spirocyclic lactones. Part B: The regioselective oxidative coupling of phenols | Semantic Scholar
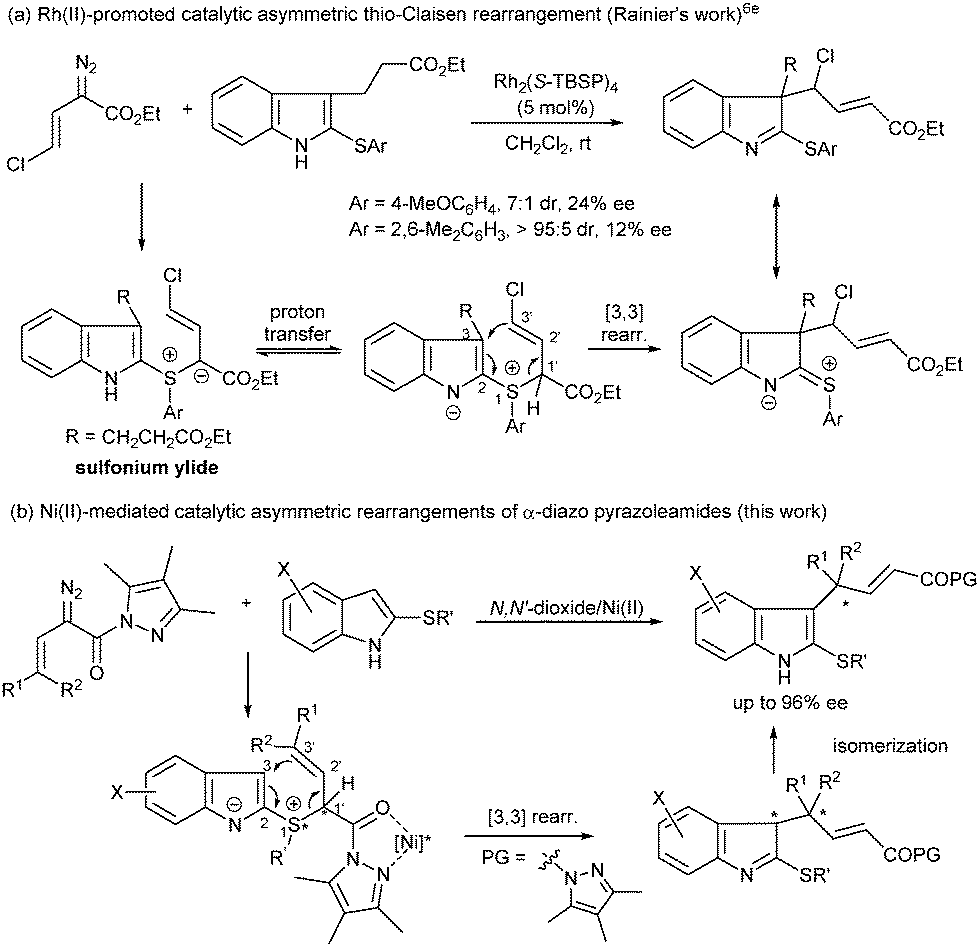
Nickel(ii)-catalyzed asymmetric thio-Claisen rearrangement of α-diazo pyrazoleamides with thioindoles - Chemical Communications (RSC Publishing)

Practical, Highly Active, and Enantioselective Ferrocenyl–Imidazoline Palladacycle Catalysts (FIPs) for the Aza‐Claisen Rearrangement of N‐para‐Methoxyphenyl Trifluoroacetimidates - Weiss - 2006 - Angewandte Chemie International Edition - Wiley Online ...

Proposed mechanism for Pd-catalysed rearrangement of allyloxypurines I. | Download Scientific Diagram

Table 1.2 from Part A: Palladium(II)-Catalyzed Enantioselective Saucy-Marbet Claisen Rearrangement of Propargyloxy indoles to Quaternary Oxindoles and Spirocyclic Lactones. Part B: The Regioselective Oxidative Coupling of Phenols . | Semantic Scholar

Table 1.4 from Part A: Palladium(II)-catalyzed enantioselective Saucy-Marbet Claisen rearrangement of propargyloxy indoles to quaternary oxindoles and spirocyclic lactones. Part B: The regioselective oxidative coupling of phenols | Semantic Scholar

Tandem Pd(II)-Catalyzed Vinyl Ether Exchange−Claisen Rearrangement as a Facile Approach to γ,δ-Unsaturated Aldehydes

![Palladium catalyzed polyhetero-Claisen rearrangement - [PDF Document] Palladium catalyzed polyhetero-Claisen rearrangement - [PDF Document]](https://reader011.staticloud.net/reader011/html5/20190224/5750a2be1a28abcf0c9d7651/bg1.png)